Abstract
BACKGROUND:
Sickle cell disease (SCD) is a group of inherited hemoglobinopathies that continues to be highly morbid and lethal. SCD-associated platelet hyperreactivity is a well-recognized contributor to the pathophysiology of the disease via complex interactions with the immune system and endothelium. Aberrant platelet bioenergetics have been implicated as a biological mechanism for SCD-associated platelet hyperreactivity, however, little is known about the impact current medical interventions (e.g., hydroxyurea [HU] and red blood cell [RBC] exchange transfusions) have on the platelet functional-bioenergetic profile. In this study we investigate the effects of hydroxyurea and RBC exchange transfusions on reprograming the platelet functional-bioenergetic profile and provide insight into biological pathways that may be amenable to intervention.
METHODS:
Platelets from sex-, race-, and aged-matched adult healthy control subjects and adult patients with homozygous SCD (HbSS), actively being treated with hydroxyurea (HU group) or RBC exchange transfusions (RBC exchange transfusion group), were isolated and washed following standard protocols. Platelet activation by flow cytometry was determined at baseline and following activation with thrombin (0.075U/ml) and ADP (1.25uM). Platelet-activated fibrinogen binding site (αIIbβIII), P-selectin, and phosphatidylserine (PS) surface marker expression (as measured by mean fluorescence intensity [MFI]) was determined with PAC-1, P-selectin, and lactadherin antibodies, respectively. The bioenergetic profile of washed platelets was determined by the 24-well format Seahorse extracellular flux analyzer. Statistical analyses were performed using the one-way ANOVA. Correlations were performed by 2-tailed nonparametric Spearman correlations and linear regression analysis with 95% confidence interval (GraphPad Software v9.1.2). Data expressed as mean plus or minus standard error of the mean (SEM). Differences were considered significant at p < 0.05.
RESULTS:
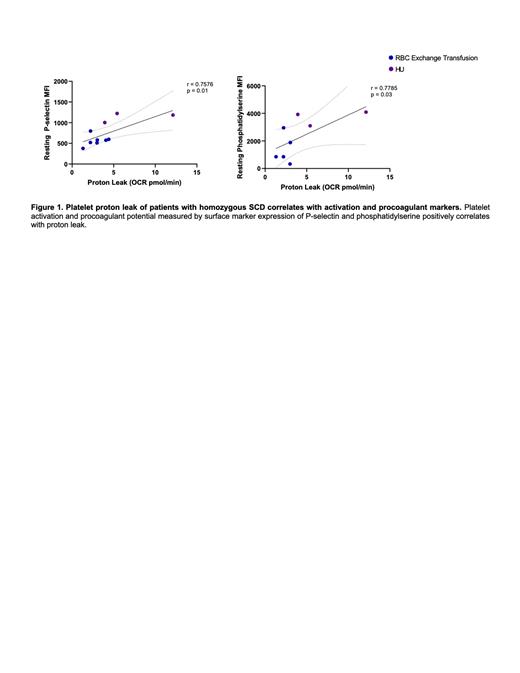
Platelets from patients in the HU group exhibited increased surface marker expression of αIIbβIII (p = 0.004), P-selectin (p = 0.003), and PS (p = 0.003) at resting conditions when compared to the RBC exchange transfusion group and healthy controls. Additionally, an increase in PS expression was seen in the HU group upon activation with ADP (p = 0.0003). No significant differences were seen in the platelet functional profile after activation with thrombin. The platelet bioenergetic profile in the HU group demonstrated an elevated proton leak (p = 0.03) when compared to the RBC exchange transfusion group. Elevated proton leak in SCD was found to have positive correlation with P-selectin and PS expression (Figure 1).
CONCLUSION:
While therapeutic interventions have improved overall outcomes in patients with SCD, adverse events continue to be a deterrent to many patients prompting the need for safer, more tolerable, and cost-effective alternatives. We have identified that while HU has little impact on the hyperreactive and procoagulant platelet phenotype in SCD, RBC exchange transfusions appear to mitigate the phenotype and reprogram the bioenergetic profile. Amongst treatment groups, a strong correlation was found between platelet activation markers (i.e., P-selectin and PS) and proton leak, suggesting an interplay between alterations in platelet bioenergetics and SCD-associated platelet hyperreactivity. Further studies are needed to elucidate the metabolic pathways that are responsible for the aberrant platelet functional-bioenergetic profile seen in SCD. These observations are important as targeting the platelet bioenergetic profile via less invasive and toxic therapeutic modalities may be equally efficacious as current interventions.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal